(Eagle News) – The Food and Drug Administration stressed that China’s Sinovac vaccines had an efficacy rate as high as 91.2 percent in the general population in Turkey, and up to 65.3 percent in Indonesia, and were therefore effective for the general population in preventing COVID-19.
FDA Director General Undersecretary Eric Domingo also assured the public that Sinovac vaccines, under the trade name Coronavac, had not exhibited any severe adverse effects in the various clinical trials, nor in the countries where these had been used.
It is therefore very safe for the public to use, and will be effective against COVID-19.
The FDA chief also assured that the vaccines are even safe for those with a history of allergies, and is even a “good option” for them.
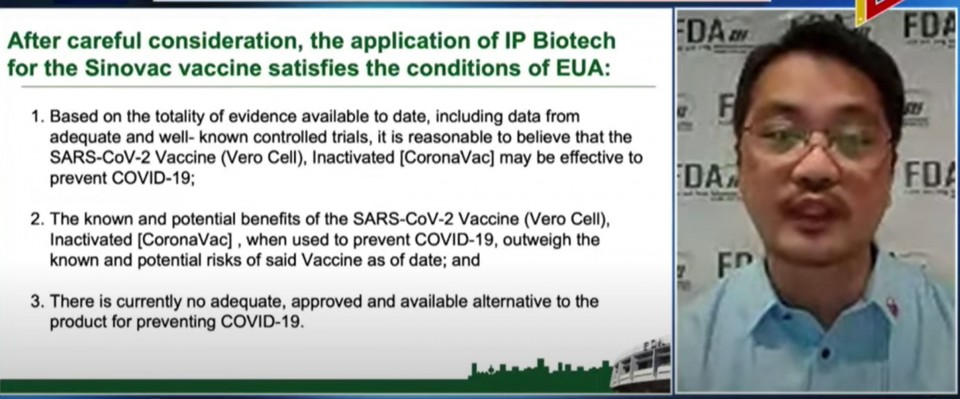
Domingo said that they based their assessment “after thorough and rigorous review of the currently available published and unpublished data by our regulatory and medical experts.”
-Benefits outweigh risks-
In giving an Emergency Use Authorization for the “COVID-19 vaccine of Sinovac Life Sciences Company known as SARS–COV-2 vaccines Cero Cell Inactivated” or Coronavac, he said that “all conditions for an EUA are present.”
He also stressed that “the benefit of using the vaccine outweighs the known and potential risks.”
But he said that for the health care workers who are more exposed to COVID-19 because of the nature of their work, it is better to give a vaccine that has a higher efficacy rate.
-Efficacy of 50.4 percent only on health care workers-
Domingo explained his reason: Sinovac vaccines had an efficacy rate of 50.4 percent for health care workers in Phase 3 clinical trials in Brazil.
“The interim data from the ongoing phase 3 trials show that when the vaccine is used on clinically healthy members of the community, aged 18 to 59, it has an efficacy rate of 65.3% to 91.2%,” the FDA chief said.
The Sinovac vaccines are not advised to be given to the elderly, Domingo said.
“Iyong 65.3, iyan po iyong nakita sa Indonesia at iyong 91.2 iyon po iyong sa Turkey sa kanilang clinical trial. However, it has a lower efficacy rate of 50.4% when used on health care workers exposed to COVID-19. Therefore, it is not recommended for use in this group,” he explained.
-No report of severe allergic reactions on Sinovac vaccines-
Domingo also observed that the Sinovac vaccine is also safe for those with allergies. This is unlike the Pfizer vaccines which have reported incidence of severe allergic reactions, sometimes even life-threatening ones.
The US Centers for Disease Control (CDC) even noted that among those given first doses of the Pfizer-BioNTech vaccines in the US as of December 23, 2020, “175 case reports were identified for further review as possible cases of severe allergic reaction, including anaphylaxis.”
“Anaphylaxis is a life-threatening allergic reaction that does occur rarely after vaccination, with onset typically within minutes to hours (3),” the US CDC said on the Pfizer-BioNTech vaccine review.
Domingo said that in the case of Sinovac, there were no reports of severe adverse reactions to the vaccines. In fact, the reactions were mostly mild – the usual mild fever and slight pain in the area where it was injected.
“We would like to inform the public that the vaccine is a good option for individuals who have allergies to component of other available vaccines such as iyong Polyethylene glycol and polysorbate, hindi po siya nagko-contain nitong mga chemicals that are usually the ones that cause an anaphylaxis and severe allergies,” Domingo said.
-Halal-certified-
“The vaccine produced by Sinovac is also certified halal by the Indonesian authorities. And the following are the salient points of the EUA and of course the EUA may be revisited later on and reviewed and revised as we get more data form their ongoing clinical trials,” he said.
Domingo said that the “the vaccine regimen recommended by our experts” for the Sinovac vaccine administration “consist of two equal standard doses of 0.5 ml each given four weeks apart.”
“The adverse events reported were transient and mostly mild to moderate similar to common vaccine reactions. No specific safety concerns were identified, but it must be noted that this only reflects limited follow-up and more adverse effects may emerged that is why close surveillance and monitoring is needed after the immunization,” he added.
China had earlier promised to send 600,000 doses of Sinovac vaccines to the Philippines, with 100,000 allocated for the country’s military.
They are expected to arrive in a matter of days. The Chinese government had asked for three days for the processing of the delivery.
Presidential Spokesperson Harry Roque on Monday, Feb. 22, said that because of the conditions set by the FDA for the EUA given to Sinovac, the National Immunization Technical Advisory Group (NITAG) will have to revise its priority schedule with respect to the Sinovac vaccines, and change the priority recipients from health care workers and the elderly to the military and the economic frontliners.
He also said that because President Rodrigo Duterte is already 75-years old, he is not advised to bjab.e given the Sinovac
The most notable figures who publicly received the Sinovac vaccines were 59-year old Indonesian President Joko WIdodo and 63-year old Hong Kong Chief Executive Carrie Lam. Both made public their vaccinations as part of their respective government’s campaign to convince the public to haev themselves immunized.
(Eagle News Service)








