
(Eagle News) — Indonesian President Joko Widodo was the first to be inoculated with China’s Sinovac vaccine against COVID-19 on Wednesday, Jan. 13, as Indonesia officially kicked off its mass vaccination drive starting with the Chinese-developed vaccines.
The vaccination of the Indonesian president was broadcast live on television.
Widodo had earlier announced that he would be the first one to get the jab to allay the public’s fears about getting vaccinated against COVID-19.
The 59-year Indonesian leader was given the Sinovac BioTech’s vaccine, CoronaVac, along with his cabinet members at the presidential Palace in Jakarta.
“This COVID-19 vaccination is important for us to break the chain of this coronavirus transmission and provide health, safety, and protection for all Indonesians,” Mr Widodo said after the vaccination.
“I don’t feel it at all,” he said with a laugh after receiving the first of the second dose of the Sinovac vaccine, CoronaVac.
Mr. Widodo, also known as Jokowi, will get the second dose at a later date.
Indonesia has already received 3 million doses of the CoronaVac vaccine developed by China’s Sinovac Biotech Ltd.
-First emergency use approval outside China
The country’s Food and Drug Administration (FDA), known as Badan POM, gave emergency use approval to the COVID-19 vaccine developed by China’s Sinovac BioTech Ltd., on Monday, which was what the Southeast Asian country waited for before starting the mass vaccination.
Indonesia is prioritizing its more than 1.3 million health workers in this vaccination program against COVID-19.
The Indonesian FDA’s interim analysis of previous late-stage trials of the Sinovac vaccine in the country said that it had an efficacy rate of 65.3 million which is above the World Health Organization’s requirement of 50 percent efficacy.
Indonesia’s emergency use approval of the Sinovac vaccine is also the first emergency use approval for the said vaccine outside China

The Muslim-majority nation’s top religious body also approved the vaccine as halal — meaning permissible under Islam — in a move that could help convince wary citizens.
Previous vaccination drives have met resistance among some segments of the country’s huge population, the world’s fourth largest.
-Mass vaccination for 182 million people in Indonesia-
Widodo said that the mass vaccination will “also help speed up the economic recovery” of the country with a population of more than 270 million.
Health workers and other at-risk groups will get priority under an ambitious plan to inoculate nearly 182 million people over the next 15 months.
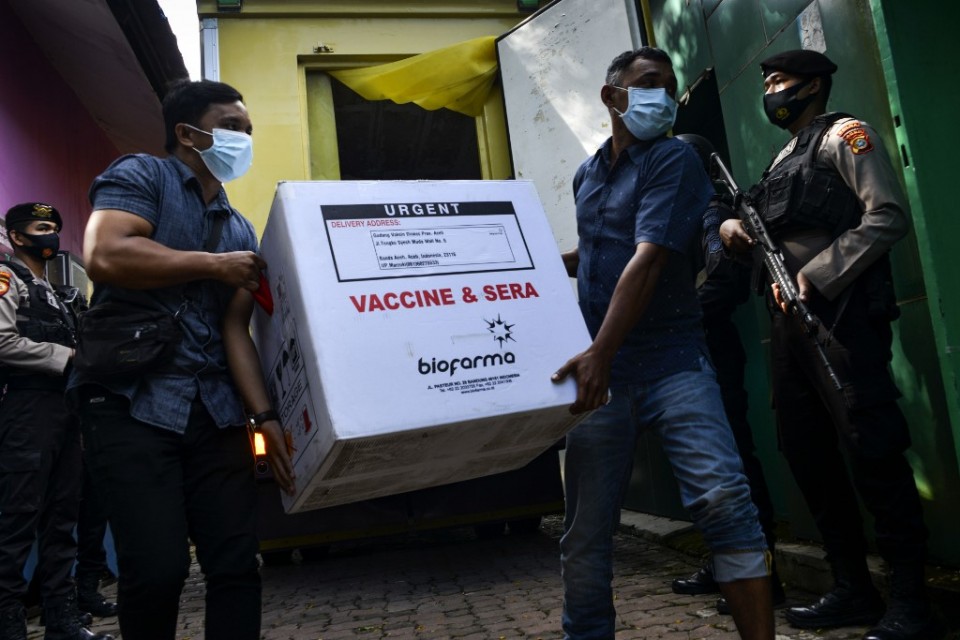
The Southeast Asian nation has already signed deals for nearly 330 million vaccine doses from a string of pharmaceutical companies including UK-based AstraZeneca, Pfizer and Chinese suppliers including Sinopharm.
It has reported nearly 850,000 Covid-19 cases and close to 25,000 deaths, but low testing rates mean the public health crisis is believed to be much bigger than the figures suggest.
The Sinovac vaccine uses chemically inactivated whole virus vaccine for COVID-19 to expose the immune system of the body, which is the more traditional type of vaccine, and requires two doses to be effective.
It does so with without risking a serious disease response.
One of its benefits also is that it can be stored in cold storage with temperatures of between 2 degrees Celsius and 8 degrees Celsius, making it more transportable in countries without ultra-cold storage facilities.
In contrast, the Pfizer and BioNTech vaccine needs to be stored under extremely cold temperatures of negative 70 degrees Celsius at least, to preserve its efficacy
(Eagle News Service with a report from Agence France Presse)







