PHL partners with Serum Institute of India which will produce vaccines
(Eagle News) — The Philippines has secured 30 million doses of the vaccine, Covovax, which was developed by Novavax Inc, a biotechnology company based in the United States, and is set to be produced by the Serum Institute of India (SII).
Palaca spokesperson Harry Roque said that this is second good news that the Philippine government has on the matter of securing COVID-19 vaccines for the country.
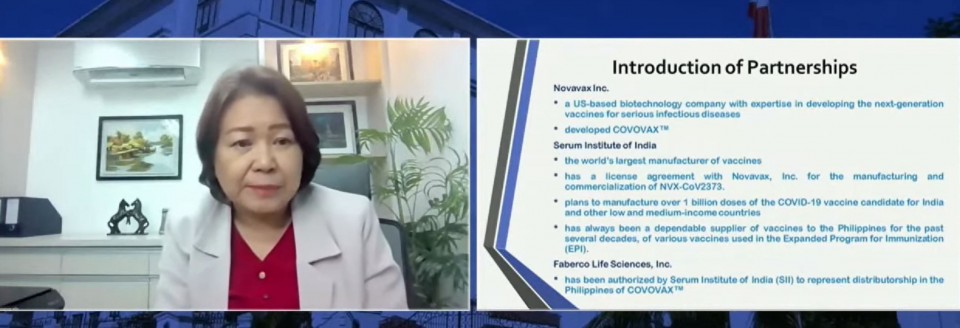
He said Philippine vaccine czar Secretary Carlito Galvez Jr., had also signed an agreement with SII and the Faberco Life Services Inc. for 13 million vaccine doses by the start of the third quarter of this year.
Roque explained that SSI is the “biggest vaccine manufacturer in the world.” SSI partnered with the US-based biotech company Novavax which first developed the Covavax vaccine.
The vaccines will be delivered by the third quarter of the year.
-Only 2 degrees Celsius to 8 degrees Celsius needed for Covovax storage-
He also noted that unline other vaccines like the one developed by Pfizer and BioNTech, the Covovax vaccine don’t need to be stored in extremely cold temperatures. The Pfizer vaccine requires a cold storage of at least negative 70 degrees Celsius.He said the Chinese vaccines like Sinovac also don’t need ultra-cold storage as the Pfizer vaccine.
He said Covavax only needs a storage of 2 degrees to 8 degrees Celsius, and chided critics whom he said have a “colonial mentality” for preferring Western-made vaccines like Pfizer.
“Ang maganda po sa Covovax, kasama po ng mga Chinese vaccines, ay kinakailangan lamang ng two degrees centigrade to eight degrees centigrade ang standard temperature sa existing cold chain system sa Pilipinas. Iyong iba po kasing brand, lalung-lalo na iyong Pfizer, ay mantakin ninyo po, negative seventy (-70) ang kinakailangan. Eh pati po sa Amerika na napakadaming nilang na-order, nasasayang po dahil from the factory to delivery, eh naku, hindi po nasusunod iyong cold chain,” Roque said.
The Palace spokesperson also said that the Philippines will have difficulties in getting the cold-chain distribution facilities of negative 70 degrees Celsius as required by Pfizer’s COVID vaccines.
“So sa mga mayroong colonial mentality na gusto ang Pfizer, well, puwede po kayong mag-antay pero ang ating warning po ay talagang diyan lang po iyan maibibigay dito sa Pilipinas sa mga major na siyudad kasi wala naman talaga tayong cold chain capacity outside of Metro Manila na -70; ang freezing po ay zero ‘no. Eh mantakin ninyo iyong -70, saan tayo kukuha niyan,” he said.
In the same press briefing, Dr. Ma. Luningning Elio-Villa, Medical Director at Faberco Life Sciences, Inc., which will represent SII in the distributorship of Covovax in the Philippines, confirmed that there is indeed a commitment already on the part of SII to supply 30 million doses of Covovax to the Philippines. Faberco will be the local company coordinator that will ensure that the vaccines will be properly received and delivered to the national government.
-Clinical trials for Covovax-
Dr. Villa said that Covovax is currently undergoing phase three clinical trials in the United Kingdom, the United States, Mexico, and India. She added that the clinical trials involve multi-racial studies to ensure the vaccines efficacy and safety across multiple races of people.
De Villa said that because Covovax only require two degrees Celsius to eight degrees Celsius, this can be easily distributed to even far-flung barangays.
“And sinabi nga ni Spox kanina na it can be stored at two to eight degrees centigrade. So kahit doon sa malalayong barangay na mayroong health facility at magkakaroon ng vaccination, kayang-kaya po, stable pa rin po ang Covovax at those regular cold chain temperatures,” she said.
“And hopefully po magiging available po ang bakuna by the third quarter and we are very optimistic na magiging maganda po ang resulta at magkakaroon din po ng emergency use authorization from UK which is a stringent regulatory authority. And of course, we hope that even WHO would give the recommendation for our product,” De Villa explained.
With the preliminary results of phase three clinical trials, of which many are nearing completion already, Dr. Villa said that she is hopeful that the delivery of the 30 million doses is expected to be completed within five to six months upon the finalization of the agreement.
(Eagle News Service)








