
(Eagle News) — Researchers recently found a promising vaccine against the deadly Ebola virus which was tested in Guinea where it was found to be 100 percent effective, according to the World Health Organization.
The vaccine called VSV-EBOV was found to be “highly effective against Ebola” based on the results from an interim analysis of the Guinea Phase II efficacy vaccine trial.
The independent body of international experts – the Data and Safety Monitoring Board – that conducted the review, advised that the trial should continue. Preliminary results from analyses of these interim data are published in the British journal, “The Lancet.”
“This is an extremely promising development,” said Dr Margaret Chan, Director-General of the World Health Organization. “The credit goes to the Guinean Government, the people living in the communities and our partners in this project. An effective vaccine will be another very important tool for both current and future Ebola outbreaks.”
While the vaccine up to now shows 100% efficacy in individuals, more conclusive evidence is needed on its capacity to protect populations through what is called “herd immunity”. To that end, the Guinean national regulatory authority and ethics review committee have approved continuation of the trial, according to a WHO news release dated July 31.
“This is Guinea’s gift to West Africa and the world,” said Dr Sakoba Keita, Guinea’s national coordinator for the Ebola response.
“The thousands of volunteers from Conakry and other areas of Lower Guinea, but also the many Guinean doctors, data managers and community mobilizers have contributed to finding a line of defense against a terrible disease.”
John-Arne Røttingen, Director of the Division of Infectious Disease Control at the Norwegian Institute of Public Health and Chair of the Study Steering Group said the ‘ring’ vaccination method adopted for the vaccine trial is based on the smallpox eradication strategy.
“The premise is that by vaccinating all people who have come into contact with an infected person you create a protective ‘ring’ and stop the virus from spreading further. This strategy has helped us to follow the dispersed epidemic in Guinea, and will provide a way to continue this as a public health intervention in trial mode,” Rottingen said.
The Guinea vaccination trial began in affected communities on 23 March 2015 to evaluate the efficacy, effectiveness and safety of a single dose of the vaccine VSV-EBOV by using a ring vaccination strategy. To date, over 4 000 close contacts of almost 100 Ebola patients, including family members, neighbors, and co-workers, have voluntarily participated in the trial.
The trial stopped randomization on 26 July to allow for all people at risk to receive the vaccine immediately, and to minimize the time necessary to gather more conclusive evidence needed for eventual licensure of the product. Until now, 50% of the rings were vaccinated 3 weeks after the identification of an infected patient to provide a term of comparison with rings that were vaccinated immediately. This now stops. In addition, the trial will now include 13 to 17-year-old and possibly 6 to 12-year-old children on the basis of new evidence of the vaccine’s safety.
“If the vaccine is effective, then we are protecting frontline workers from the virus,” WHO said.
Bertrand Draguez, Medical Director at Médecins sans Frontières announced that they would also be conducting a trial of the same vaccine on frontline workers in parallel with the ring vaccination.
“These people have worked tirelessly and put their lives at risk every day to take care of sick people. If the vaccine is effective, then we are already protecting them from the virus. With such high efficacy, all affected countries should immediately start and multiply ring vaccinations to break chains of transmission and vaccinate all frontline workers to protect them,” Draguez said.
The trial is being implemented by the Guinean authorities, WHO, Médecins sans Frontières (MSF) and the Norwegian Institute of Public Health, with support from a broad partnership of international and national organizations.

“This is a remarkable result which shows the power of equitable international partnerships and flexibility,” said Jeremy Farrar, Director of the Wellcome Trust, one of the funders of the trial. “This partnership also shows that such critical work is possible in the midst of a terrible epidemic. It should change how the world responds to such emerging infectious disease threats. We, and all our partners, remain fully committed to giving the world a safe and effective vaccine.”
“This record-breaking work marks a turning point in the history of health R&D,” said Assistant Director-General Marie-Paule Kieny, who leads the Ebola Research and Development effort at WHO.
“We now know that the urgency of saving lives can accelerate R&D. We will harness this positive experience to develop a global R&D preparedness framework so that if another major disease outbreak ever happens again, for any disease, the world can act quickly and efficiently to develop and use medical tools and prevent a large-scale tragedy.”
The vaccine VSV-EBOV was developed by the Public Health Agency of Canada. The vaccine was licensed to NewLink Genetics, and on 24 November 2014, Merck & Co., Inc and NewLink Genetics Corp. entered into an exclusive worldwide licensing agreement wherein Merck assumed responsibility to research, develop, manufacture, and distribute the investigational vaccine. Financial support was provided by the Canadian and US Governments, among others.
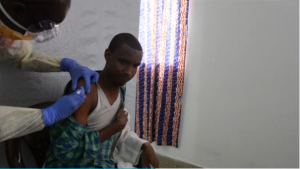
A ring vaccination protocol was chosen for the trial, where some of the rings are vaccinated shortly after a case is detected, and other rings are vaccinated after a delay of 3 weeks. This is an alternative to using a placebo by providing a randomized control group for comparison but at the same time ensures that all contacts are vaccinated within the trial.
The trial design was developed by a group of experts from Canada, France, Guinea, Norway, Switzerland, United Kingdom, United States, and WHO. The group included Professor Donald A. Henderson of John Hopkins University, who led the WHO smallpox eradication effort by using the ring vaccination strategy.
The Guinea Ebola vaccine trial is the coordinated effort of many international agencies. WHO is the regulatory sponsor of the study, which is implemented by the Ministry of Health of Guinea, WHO, Médecins sans Frontières (MSF), EPICENTRE and the Norwegian Institute of Public Health.
The trial is funded by WHO, with support from the Wellcome Trust, the United Kingdom Department for International Development, the Norwegian Ministry of Foreign Affairs to the Norwegian Institute of Public Health through the Research Council of Norway, the Canadian Government through the Public Health Agency of Canada, Canadian Institutes of Health Research, International Development Research Centre and Department of Foreign Affairs, Trade and Development and MSF.
The trial team includes experts from The University of Bern, the University of Florida, the London School of Hygiene and Tropical Medicine, Public Health England, the European Mobile Laboratories among others. (based on a WHO news release)






