(Eagle News) — The COVID-19 vaccine Moderna has already applied for Emergency Use Authorization (EUA) with the Food and Drug Administration (FDA).
Zuellig Pharmaceuticals filed the application for EUA, on behalf of Moderna. The application came more than a month before the scheduled arrival of the initial doses of Moderna vaccines. The first batch of Moderna vaccines are expected to arrive on June 15.
In an earlier interview, FDA director general Eric Domingo said that the EUA for Moderna could be processed much faster as it already has an EUA issued by the United States FDA. It has also been approved for use in the European Union by the European Medicines Agency (EMA).
“Pag meron ng EUA sa mga FDA counterpart natin sa US at EU na very stringent or very strict, pag meron nan approval na ganoon, medyo mas mabilis nang konti, : Domingo said in an earlier exclusive interview.

The latest EUA to be issued by FDA was for Covaxin which was developed by Bharat Biotech Inc., in India.
The vaccine was given EUA last April 19, the same day that the FDA issued the EUA for Johnson and Johnson’s Janssen
Covaxin can be stored at ordinary refrigerator temperatures of between 2 degrees Celsius to 8 degrees Celsius.
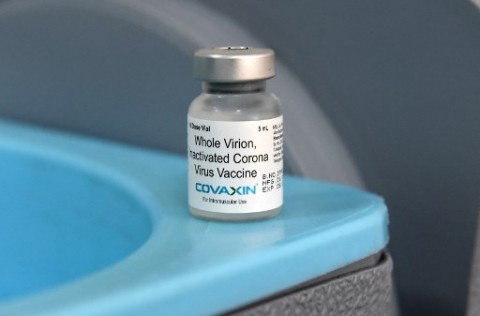
Covaxin is a whole virion, Inactivated Corona Virus Vaccine. It is “presented as Single dose (0.5 mL) and multidose (5 mL and 10 mL) in transparent vial (USP type I glass) with a stopper (Bromobutyl rubber) and a flip-off plastic cap with aluminium seal. Each vial of single dose contains 0.5 mL, each vial of multidose contains 10 doses (5 mL) and 20 doses (10 mL) respectively.”
The vaccine is to be administered for people aged 18 years and older.
-6 vaccines issued with EUA in PHL-
So far, the FDA has already issued EUAs to six COVID-19 vaccines.
● Pfizer-BioNTech COVID-19 Vaccine (BNT162b2)
● ChAdOx1-S [recombinant] (COVID-19 Vaccine AstraZeneca)
● SARS-CoV-2 Vaccine (Vero Cell), Inactivated (CoronaVac)
● Gam-COVID-Vac (Sputnik V)
● Ad26.COV2-S [recombinant] (Janssen COVID-19 Vaccine)
● Whole Virion Inactivated Corona Virus (Covaxin)
“Pfizer-BioNTech COVID-19 vaccine is a mRNA vaccine; COVID-19 Vaccine AstraZeneca, Sputnik V, and Janssen COVID-19 Vaccine are non-replicating viral vector vaccines; and CoronaVac and Covaxin are inactivated vaccines. All are administered in two doses within an interval of a few weeks except for Janssen COVID-19 Vaccine which is administered as a single-dose,” the FDA said.
(Eagle News Service)








