Bexovid and Paxlovid, with the generic name Nirmatelvir, could reduce hospitalization and deaths by around 90 percent, says Palace
(Eagle News) – The Philippine Food and Drug Administration has given a compassionate special permit to the anti-Covid-19 drug Bexovid, the first generic version of the Pfizer antiviral treatment drug Paxlovid.
This is good news for Filipinos since this would mean treatment for COVID-19 would be more accessible to ordinary Filipinos since this generic drug is cheaper that Pfizer’s Paxlovid, according to Malacanang.
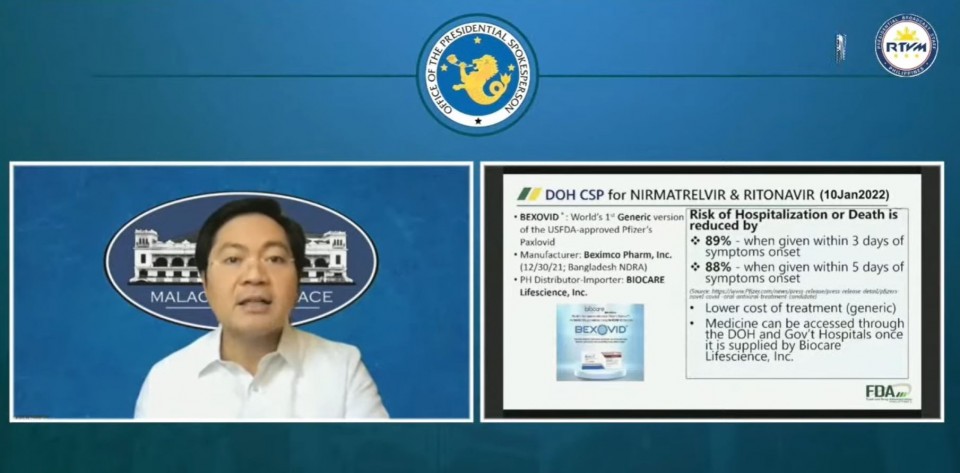
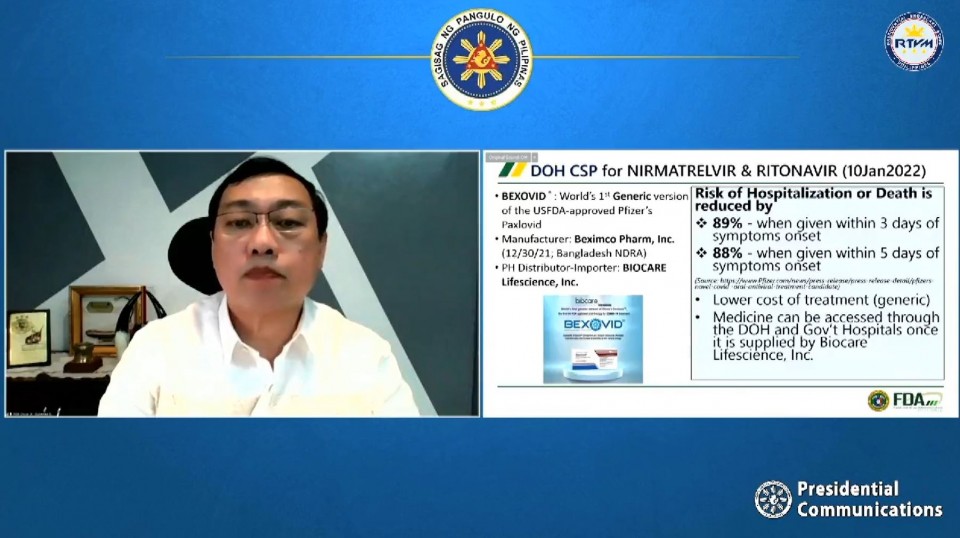
This anti-viral drug could be used by COVID-19 patients in the first three days of showing of symptoms, and is seen to reduce hospitalization or death by 89 percent, according to acting Presidential Spokesperson Cabinet Secretary Karlo Alexei Nograles.
-Lower cost of treatment for COVID-19-
“Ano po ang implikasyon nito? Magiging mas mura ang gamutan at maa-access ang gamot na ito sa DOH (Department of Health) at government hospitals. Mababawasan ng Bexovid ang risk ng hospitalization or death by 89% kung maibibigay sa pasyente sa loob ng unang tatlong araw ng sintomas,” he said.

Health Undersecretary Leopoldo Vega said that the presence of antiviral treatment or anti-COVID-19 drugs this year gives hope for those who get infected with the disease.
“Una nito ay itong antiviral drug na nagawa po ng Pfizer at ang generic name nito is Nirmatelvir. At ito ay unang nakita po at na-examine at na-research doon sa Pfizer, sa USA, at nakita nila sa data ho nila sa lahat ng mga pasyenteng nabigyan po nito, nag-reduce ang kanilang hospitalization and death to about 90%,” he said.
Nirmatelvir is the generic name of the Pfizer drug Paxlovid.
In December 22 last year, the U.S. Food and Drug Administration issued an “emergency use authorization (EUA) for Pfizer’s Paxlovid (nirmatrelvir tablets and ritonavir tablets, co-packaged for oral use) for the treatment of mild-to-moderate coronavirus disease (COVID-19) in adults and pediatric patients (12 years of age and older weighing at least 40 kilograms or about 88 pounds)”

They are recommended for those who tested positive for COVID-19 and “who are at high risk for progression to severe COVID-19, including hospitalization or death.”
The US FDA said “Paxlovid is available by prescription only and should be initiated as soon as possible after diagnosis of COVID-19 and within five days of symptom onset.”
-Bexovid, Paxlovir, Monupiravir — anti-COVID oral drugs available for affected population-
These anti-COVID-19 drugs — Paxlovid and Bexovid – are in addition to the first antiviral drug that came out in the market, Molnupiravir, manufactured by the pharmaceutical firm, Merck, Vega said.
He said the Molnupiravir drug could reduce hospitalization and death by 30 to 40 percent based on recent studies.
“Ang unang antiviral kasi na lumabas sa market, sa merkado ay iyong Molnupiravir, isa din iyong antiviral drug na ginawa ng Merck. Pero ngayon sa kakalabas ngayon ng mga resulta, mga 30 to 40% ang kaniyang reduction in hospitalization and death,”Vega said in a Palace press briefing on Tuesday, January 11.
Vega said that the Pfizer drug Paxlovid and its generic version Bexovid were more effective in preventing COVID-19 hospitalization and deaths, especially for the high-risk members of the population – the elderly and those with comorbidities.
“So itong Bexovid ngayon ay ito iyong trade name ng Nirmatelvir o sa US, ito iyong Paxlovid. Itong antiviral na po ito, ito ay maibigay sa mga pasyenteng with mild and moderate lalung-lalo na sa mga high-risk, ano, kasi talagang bababa ang kaniyang hospitalization and even death,” Vega explained.
The DOH official said that the availability of these anti-COVID-19 drugs this year is a good development giving hope to many. This intervention is in addition to the intensive COVID-19 vaccination being done already by the Philippine government.
“Ngayon, itong Paxlovid to be given in five days at nasa isang package nga iyan, itong Bexovid o Paxlovid for the next five days. Ito ay talagang makakabigay ng medyo pag-asa sa ating 2022 lalung-lalo na itong pagharap natin sa COVID-19. At least mayroon na tayong mga gamot aside from the vaccination that we are pursuing,” he said.
“At least ngayon, nailagay tayo sa isang situation na mayroon tayong pag-asa or hope compared noong 2021 at 2020 kasi mayroon na tayong mga bagong gamot na puwede nating makuha sa 2022,”Vega stressed.
The FDA gave an EUA to the COVID-19 Drug Molnupiravir on December 22, 2021.
“COVID-19 Drug Molnupiravir 800mg (four 200mg capsules) should be administered orally every 12 hours for five (5) days. This should be 3 given as soon as possible after a diagnosis of COVID-19 has been made and within 5 days of symptom onset,” the FDA said.
The drug, however, is “not recommended for women with childbearing potential, pregnant and lactating women.”
On Monday, January 10, Philippine FDA OIC Director General Oscar Gutierrez Jr., announced the approval of the compassionate special permit for Bexovid.
“Pfizer granted po a royalty-free license for the antiviral combination drug ng Medicines Patent Pool, a non-profit organization based in Geneva and backed by the United Nations. It allowed the manufacturer or manufacturers in least developed countries, like Bangladesh, to take out a sub-license,” Garcia told President Duterte during the latter’s Talk To The People.
He said that Beximco Pharmaceuticals of Bangladesh was the holder of the FDA Philippines Foreign GMP Clearance.
“Inaasahan po na mas mura ang cost of treatment dahil generic drug po ang Bexovid. Ang Biocare Lifescience, Inc. po ang magiging supplier ng DOH,” Garcia said.
“The treatment is given as two tablets of Nirmatrelvir and one tablet of Ritonavir together twice a day for five days. Indicated po siya for aged 12 at pataas na may mild to moderate infection,” he said.
Gutierrez said that the the FDA is already talking with Pfizer regarding for the EUA application of Paxlovid.
“Natuloy po ‘yung aming meeting with the Pfizer team last January 7, at most likely po they will file their EUA application on the last week of January,” he said.
The FDA has also given an EUA to another anti-COVID drug, Casirivimab + Imdevimab (COVID-19 Drug Casirivimab + Imdevimab) with the brand name “Ronapreve” But this is administered intravenously, and not orally.
“This should be administered together (Casirivimab 600mg and Imdevimab 600mg) as a single intravenous infusion for the treatment of confirmed mild to moderate COVID-19 in patients aged 12 years and older and weighing at least 40 kg that do not require supplemental oxygen for COVID-19 and who are at high risk of progressing to severe COVID-19,” the revised FDA EUA said.
(Eagle News Service)