FDA gives green light after PHL vaccine expert panel recommendation on Sinovac use for senior citizens, citing its safety and efficacy

(Eagle News) – The Philippines’ Food and Drug Administration approved on Wednesday, April 7, the use of Sinovac vaccines for the elderly.
FDA Director-General Undersecretary Eric Domingo said that they approved the Sinovac vaccine with the brand name Coronavac for use in the elderly or those aged 60 and above after the recommendation made by the Philippine vaccine expert panel on Sinovac’s use for the elderly.
“After considering the recommendation of the experts and the current situation of high COVID-19 transmission and limited available vaccines, the FDA is allowing the use of Sinovac on senior citizens,” FDA’s Domingo said in a statement on Wednesday, April 7.
But Domingo said that there should be an evaluation first done on the health condition of the senior citizen who would be given the vaccine.
The Department of Health (DOH) said there should be a “stringent evaluation of the person’s health status and exposure risk.”
“The decision to allow the use of the vaccine for seniors came about upon the recommendation of the Department of Science and Technology’s Vaccine Expert Panel, and considering the limited availability of vaccines and the growing need to protect seniors amid the increasing number of COVID-19 cases in the country,” a DOH statement said.
“However, the DOH and FDA emphasized that the vaccination of senior citizens using CoronaVac should strictly be preceded by careful evaluation of the person’s health status and exposure risk. There is phase I/II safety and immunogenicity data for seniors but efficacy data is not yet sufficient to establish vaccine efficacy,” it added.
Both the DOH and FDA stressed that “while current efficacy data for Senior Citizens from Phase III trials is insufficient, the benefits of using the vaccine for this particular group outweigh its risks, and more scientific data on use for senior citizens may soon become available.”
The DOH also urged the public–especially senior citizens and persons with comorbidities–to register for and participate in the government’s COVID-19 vaccination program to afford themselves the added layer of protection that it can provide.
Before this announcement by the FDA and the DOH, Dr. Nina Gloriani, head of the Philippine vaccine expert panel, reiterated the panel’s recommendation for the FDA to approve Sinovac for the elderly, citing evidence on its safety and efficacy.
Dr. Gloriani, in a Laging Handa press briefing before noon on Wednesday, April 7, said she himself already had the two doses of the Sinovac vaccine, with the brand name Coronavac. She said apart from the mild pain at the injection site, she did not feel anything else. She said that after a few hours, the pain subsided. The second dose of the Sinovac vaccine is given 28 days after the first dose.
“Ako siguro ang living example ng isang senior na nakakuha ng bakuna pero mild na mild lang ang naramdaman ko,” she said.
“Maganda ang kanyang safety profile,” she said noting that those who got the Sinovac vaccines only had mild to moderate side-effects, citing data from trials in Brazil and in China, and even from the experience of other senior citizens in the Philippines which received SInovac shots.
Dr. Gloriani, 67, said aside from pain in the injection site, some of those who had received the Sinovac jabs experienced only mild headache or flu-like symptoms, but nothing more serious than this.
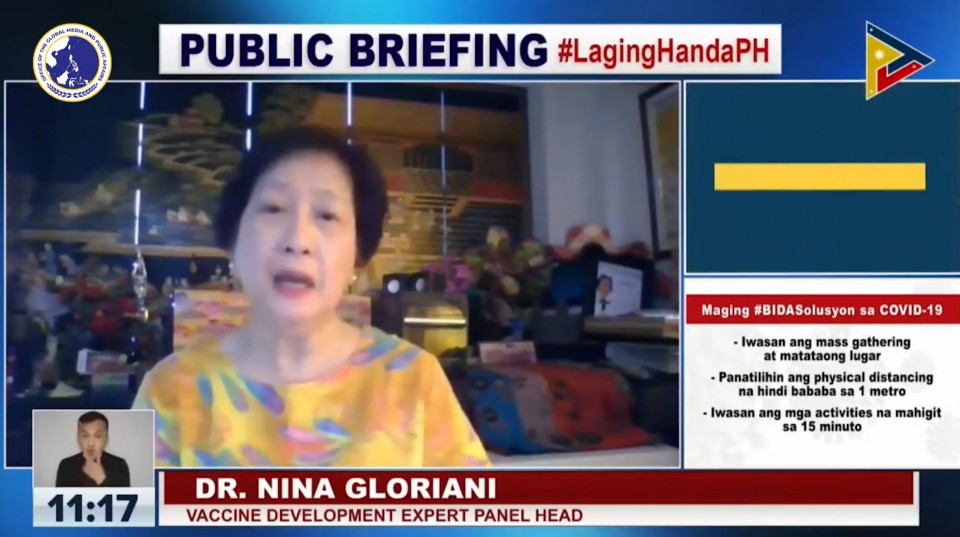
“Nasa pandemya tayo ngayon at hindi tayo puedeng mag-delay nang mag-delay dahil ang bakunang iniintay natin ay hindi pa dumarating,” she said on Wednesday as she urged the FDA to approve Sinovac’s Coronavac for use in the elderly.
Before the announcement from FDA allowing Sinovac to be used for the elderly, it had only approved the administration of AstraZeneca vaccine for those aged 60 and above. Senior citizens, however, can opt to get Sinovac vaccines if they want to, provided there is a signed waiver on their part.
Dr. Gloriani said that the antibodies that were developed among the elderly who had the Sinovac jabs, were also the same antibodies that those in the younger population had after getting Sinovac.
This meant that those given Sinovac’s Coronavac would not get severe COVID-19, even if they contract the disease.
-AstraZeneca supplies tight as export controls in EU, India implemented-
AstraZeneca has been having problems with supplies recently, that even those committed under the COVAX Facility of the World Health Organization (WHO) were having delays in its delivery affecting countries which had been promised the AstraZeneca jabs.
This was because the European Union had implemented export controls on AstraZeneca vaccines coming from EU as EU-member nations experienced a spike in cases which was higher than their previous recorded peak.
India’s recent decision to slow COVID-19 vaccine exports is also another contributory factor.
Dubbed the “pharmacy of the world,” India announced last week it was putting the brakes on exporting Covid-19 vaccines as it battled a new wave of infections and a faltering inoculation drive at home. Among those affected by this decision are Oxford/AstraZeneca jabs produced by the Serum Institute of India.
The Philippines so far has 2 million Sinovac doses — 1 million of which were donated by China delivered in two batches on Feb. 28 and March 24), while the other 1 million doses were bought by the Philippine government at P700 million which arrived on March 29.
WHO’s COVAX Facility, on the other hand, has so far delivered only 525,600 doses of AstraZeneca vaccine. The deliveries originally scheduled this week and earlier in March had been delayed.
WHO country representative Dr. Rabindra Abeyansinghe said that the 929,000 doses of AstraZeneca vaccines which should have been delivered this March or first week of April had been affected by the AstraZeneca tight supplies.
“The agreed quantity which was 920,000 vaccine doses will come but because of the shortage, what we have been informed is that we may need to expect the reduced quantity which may come over the next few weeks,” he said in a Palace briefing on Monday, April 5, 2021.
“But eventually as production peaks up, we will deliver on the 20% stock, 20% of vaccines to cover 20% of the population. So this is the agreement that the COVAX has with the Philippine government and we are still committed to do that. We believe that when production peaks up in the late second quarter to fourth quarter, we will be able to deliver.”
The administration of the AstraZeneca doses is about 12 weeks or three months apart, which is longer than Sinovac’s 28-day interval.
(with a report from Agence France Presse)







